By IDSE News Staff
The FDA approved berdazimer topical gel, 10.3% (Zelsuvmi, Ligand Pharmaceuticals) for the treatment of molluscum contagiosum in adults and children 12 months of age and older.
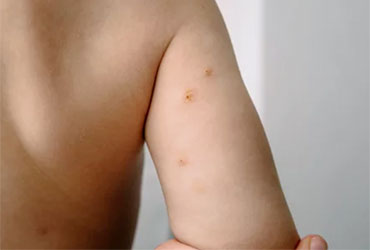
Berdazimer, a nitric oxide releasing agent, is the first novel drug for the treatment of molluscum infections. Nitric oxide has been shown to have antiviral properties, but the mechanism of action of berdazimer for the treatment of molluscum contagiosum is unknown. Nevertheless, berdazimer's efficacy was
