Vaccination with either of the currently available respiratory syncytial virus (RSV) vaccines was not associated with an increased risk for new-onset or recurrent atrial fibrillation (AFib), according to a recent study (Clin Infect Dis 2024 Dec 26. doi:10.1093/cid/ciae649).
When the FDA approved Arexvy (GSK) and Abrysvo (Pfizer) in May 2023, clinical trials demonstrated AFib events arose more frequently among the vaccine group compared with the control group.
Although these trials were insufficient in determining a causal link between AFib and RSV vaccination, the findings raised concerns about potential cardiac risks, according to Scott Roberts, MD, the associate medical director of infection prevention at Yale New Haven Health, in Connecticut. In fact, a CDC study published in May reported that a striking 14% of recipients of Arexvy or Abrysvo ages 60 years and older experienced AFib within 42 days of vaccination (MMWR Morb Mortal Wkly Rep 2024;73[21]:489-494).
“This is problematic because the RSV vaccine is specifically marketed toward older individuals—with the current CDC Advisory Committee on Immunization Practices recommendation being for people over 75 or ages 60 to 74 with conditions that place them at high risk—and this is the age group where AFib causes the most disease,” Dr. Roberts said.
However, the study provided reassurance about the vaccine’s cardiac safety. The large retrospective study by Morgan Birabaharan, MD, and his colleagues at the University of California, San Diego and Case Western Reserve University School of Medicine, in Cleveland, analyzed nearly 100,000 RSV vaccine recipients, comparing them with people vaccinated against influenza or tetanus-diphtheria-pertussis (Tdap). After adjusting for demographics and comorbidities, they found no significant increase in the risk for new-onset AFib among RSV vaccine recipients.
For patients with a history of AFib, RSV vaccine recipients reported fewer AFib recurrences than those vaccinated against influenza or Tdap, according to Dr. Birabaharan. Within 42 days after vaccination, 31.8% of RSV vaccine recipients with prior AFib experienced a recurrence, compared with 35.6% after Tdap and 35.0% after influenza vaccination.
Some experts, like Kathleen Stergiopoulos, MD, PhD, a noninvasive cardiologist at St. Francis Hospital & Heart Center, in Roslyn, N.Y., pointed to inflammation triggered by vaccination as a possible cause for recurrent AFib in susceptible individuals.
“The more active your immune system is, the more likely you are to develop AFib,” Dr. Stergiopoulos, who is unaffiliated with the study, told Infectious Disease Special Edition. “Because vaccines activate your immune system, it may be that in some older patients, particularly those with comorbidities—such as hypertension, diabetes, valvular heart disease and atherosclerosis—or known history of AFib, vaccination triggers an inflammatory process, which could stimulate the onset of AFib.”
Because the study demonstrates that when compared with the Tdap and influenza vaccines, the RSV vaccine was not associated with an increased risk for new-onset or recurrent AFib, “it likely means the initial AFib increase after RSV vaccination observed in clinical trials was incidental and due to chance, rather than actually being due to an underlying mechanism with vaccination,” Dr. Roberts explained. “This study speaks to the purpose and utility of post-marketing surveillance—it can help to answer important questions that arise in initial, smaller vaccine trials.”
While the study included vaccine recipients who were 60 and older, current guidelines recommend the RSV vaccine primarily for those 75 and older or those older than 60 with comorbidities.
“It would be worthwhile to redo the study with this narrower patient population, since the indications of the RSV vaccine have changed since we conducted the study,” said Dr. Birabaharan, the lead study author and third-year infectious disease fellow at UC San Diego.
The researchers plan to extend their analysis of AFib risks beyond the 42-day post-vaccination window, exploring potential events at 90 days and even six months in future studies.
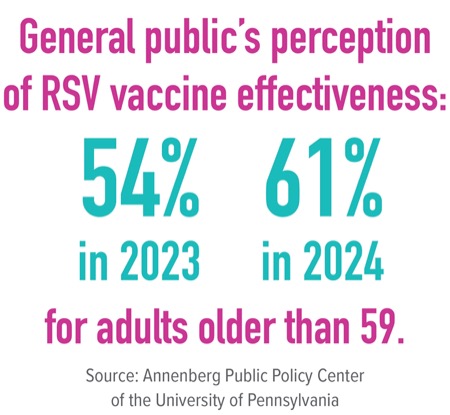
The general public’s perception of RSV vaccine effectiveness for adults older than 59 has increased over the past year, from 54% in 2023 to 61% in 2024, a survey from the Annenberg Public Policy Center of the University of Pennsylvania found. Despite this, concerns about side effects deter some from vaccination, according to Dr. Birabaharan.
“Hopefully our paper reassures patients and physicians that the RSV vaccine is safe to administer to adults who qualify regardless of AFib history, and might encourage the vaccine’s broader use,” Dr. Birabaharan said.
Drs. Birabaharan, Roberts and Stergiopoulos reported no relevant financial disclosures.
This article is from the February 2025 print issue.