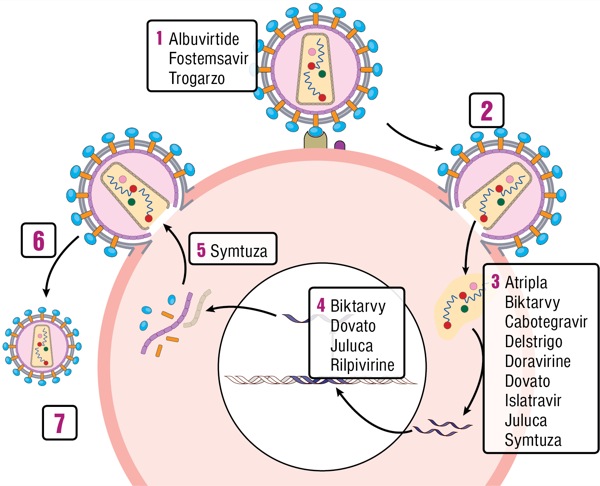
While simple, well-tolerated, effective antiretroviral therapy (ART) has been the standard of care for patients with HIV for years, the addition of new agents continues to improve therapeutic options. The central themes of advancement in HIV therapy include:
- simplified regimens;
- development of new agents for patients with multiclass resistance;
- evaluation of 2-drug regimens to decrease cumulative drug exposure; and
- implementation of novel administration strategies, such as long-acting injectables and drug-eluting implants.
In this article, we summarize newly approved medications and discuss their role in the armamentarium of modern ART. We then review medications in the ART pipeline that hold the most promise to affect future treatment.
What new drugs have been approved?
Individual Agents
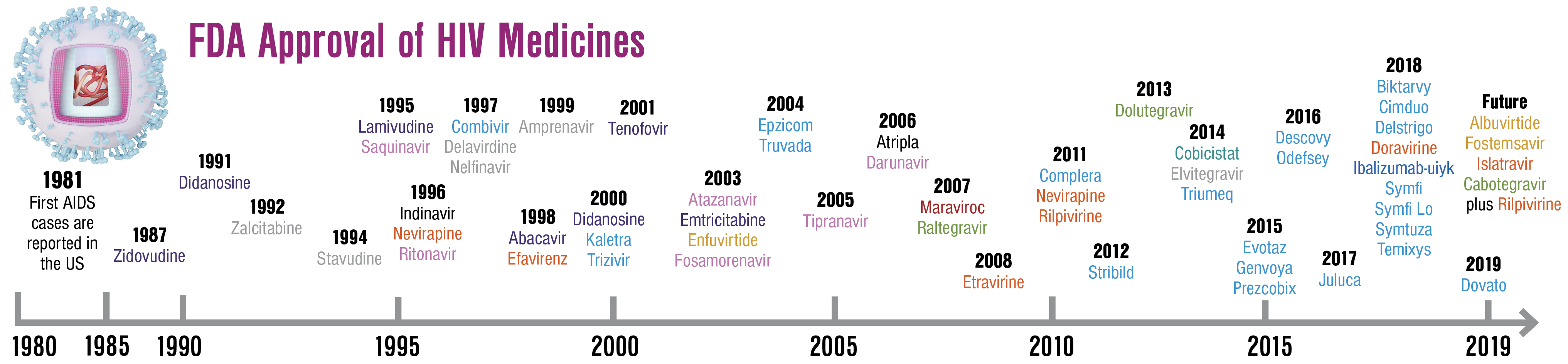
Doravirine Approved in August 2018, doravirine (DOR; Pifeltro, Merck) is a novel once-daily non-nucleoside reverse transcriptase inhibitor (NNRTI) that is effective in both treatment-naive and treatment-experienced patients. In the DRIVE-FORWARD trial of treatment-naive participants, DOR was noninferior to ritonavir-boosted darunavir (DRV/r) at 48 weeks of therapy when both were combined with 2 nucleoside reverse transcriptase inhibitors (NRTIs).1 In a subsequent analysis at 96 weeks of treatment, DOR was found to be superior due to a higher number of treatment discontinuations in the DRV/r arm.2 In the DRIVE-AHEAD double-blind, randomized trial, coformulated DOR-lamivudine-tenofovir disoproxil fumarate (DOR/3TC/TDF) was shown to be noninferior to coformulated efavirenz-emtri- citabine-TDF (EFV/FTC/TDF).3
The efficacy of DOR/3TC/TDF for the maintenance of virological suppression was evaluated in the DRIVE-SHIFT trial, an open-label switch study of treatment-experienced patients with virological control on a regimen of 2 NRTIs plus either a boosted protease inhibitor (PI), cobicistat-boosted elvitegravir, or an alternative NNRTI. DOR/3TC/TDF was found to be noninferior to staying on the baseline regimen, with approximately 94% of participants maintaining viral suppression in both arms at week 24.4
These trials have demonstrated a number of advantages for DOR. Compared with other NNRTIs, DOR has a distinct resistance pathway, retaining activity against viruses with the K103N or Y181C mutations.5 Second, unlike rilpivirine (RPV) it can be taken with or without food, and is not affected by concomitant use of proton pump inhibitors. Finally, it is generally well tolerated, with fewer central nervous system side effects and a lower incidence of drug rash than EFV. When compared with DRV/r, DOR regimens were associated with lower rates of diarrhea and more favorable lipid parameters.6
Of note, DOR has not been compared directly with integrase strand transfer inhibitors (INSTIs), which remain recommended first-line therapy in the United States. For patients who have adverse events on INSTIs, DOR may be the best available NNRTI for patients seeking alternative treatment.
Trogarzo In March 2018, ibalizumab-uiyk (IBA; Trogarzo, Theratechnologies) became the first approved medication in the post–attachment inhibitor class of antiretrovirals. Administered as a parenteral infusion every 2 weeks, it is a humanized immunoglobulin G4 monoclonal antibody that blocks CD4 receptors on the cell surface, impairing viral entry. The target population for this medication is patients with a long history of ART exposure with multiclass resistance. In a single-group, open-label phase 3 study of 40 ART-experienced patients with treatment failure and multidrug-resistant HIV, 43% of participants receiving IBA with an optimized background regimen achieved a viral load of less than 50 copies/mL, and 50% achieved a viral load less than 200 copies/mL at 24 weeks. The most common side effect was diarrhea (20%), and one patient had a severe adverse event that was attributed to immune reconstitution inflammatory syndrome.7
Biweekly parenteral infusion and cost are major barriers, so use is generally limited to patients with high-level drug resistance who have difficulty achieving viral suppression with oral therapy alone. For this small subset of patients, however, IBA is an important addition to our options for salvage therapy.
Single-Tablet, 2-Drug Combinations
Long-held dogma that effective ART must consist of 3 active antiretrovirals has been challenged by several recent studies suggesting that 2-drug therapy may be adequate for selected combinations. Two single-tablet, 2-drug regimens have been approved.
Single-Tablet, 3-Drug Combinations
Delstrigo With the approval of DOR in August 2018, the release of a DOR-based single-tablet regimen followed. DOR/3TC/TDF (Delstrigo, Merck) will be an excellent choice for patients who are switching to DOR due to adverse effects of first-line therapy and/or traditional NNRTI mutations, and who do not have baseline NRTI resistance. One potential limitation is the inclusion of TDF, as many providers now prefer TAF for its more favorable bone and renal profiles.
Biktarvy Approved in February 2018, coformulated bictegravir-FTC-tenofovir alafenamide (BIC/FTC/TAF; Biktarvy, Gilead) has rapidly become a preferred first-line regimen for both treatment-naive and treatment-experienced patients. In 2 large phase 3, randomized trials, BIC/FTC/TAF was noninferior to both dolutegravir (DTG) plus FTC/TAF and DTG/abacavir (ABC)/3TC as initial therapy in treatment-naive participants.8,9 BIC/FTC/TAF also demonstrated effectiveness as a switch regimen for patients with virological control on both PI-based therapy and DTG/ABC/3TC.10,11 Similar to DTG, BIC has a high barrier to resistance.12
Symtuza Approved in July 2018, darunavir-cobicistat-emtricitabine-tenofovir alafenamide (DRV/c/FTC/TAF; Symtuza, Janssen) is the first PI-based single-tablet regimen. This coformulated medication has proven to be effective in both treatment-naive and -experienced individuals. In the AMBER trial of treatment-naive participants, DRV/c/FTC/TAF was noninferior to DRV/c plus FTC/TDF, and no patients in either group developed PI resistance. Consistent with the growing body of literature comparing TAF and TDF, individuals on DRV/c plus FTC/TDF had higher rates of proteinuria and a lower bone mineral density compared with those on DRV/c/FTC/TAF.15 In the EMERALD trial, a large switch study for treatment-experienced patients, more than 1,000 participants on a boosted PI plus FTC/TDF were randomly assigned to receive DRV/c/FTC/TAF or remain on their baseline regimen. The DRV/c/FTC/TAF arm was noninferior with regard to maintenance of viral suppression and adverse events.16
Protease inhibitors have long posed a treatment paradox in clinical practice. Due to their high barrier to resistance, PI-based regimens are historically favored in patients who struggle with medication adherence, many of whom have NNRTI resistance mutations. However, PI-based therapy previously required 2 or 3 separate tablets, adding pill burden to their barriers to adherence. DRV/c/FTC/TAF is an effective, well-tolerated single-tablet regimen that fills an important void in our treatment armamentarium. The PI-based regimens are no longer recommended as first-line therapy in the Department of Health and Human Services (HHS) HIV treatment guidelines. However, for patients who have side effects to INSTIs, DRV/c/FTC/TAF will be an effective alternative.17
Symfi In 2006, the approval of EFV/FTC/TDF (Atripla, Gilead) as the first single-tablet regimen was a transformational moment in ART, and for many years, EFV/FTC/TDF was the most commonly prescribed ART regimen worldwide. EFV/3TC/TDF (Symfi, Symfi Lo, Mylan) is a generic single-tablet regimen that is considered interchangeable with EFV/FTC/TDF by the World Health Organization and is being sold at a markedly reduced cost.
Although a major limitation to any EFV-based regimen is the well-described central nervous system side effect profile,13 the ENCORE-1 trial showed that when compared with a standard dose of EFV (600 mg), a lower dose (400 mg) is equally effective with fewer side effects.14 This finding is relevant because Symfi Lo is an alternative coformulation of EFV/FTC/TDF that includes the lower dose of EFV.
Although use will likely be limited in the United States where INSTI-based therapy is standard of care, Symfi Lo may be better tolerated than Symfi, and could have a major impact on the cost of care in resource-limited settings.
Dovato Coformulated DTG/3TC (Dovato, ViiV Healthcare) was approved in April 2019, making it the second complete regimen consisting of a single-tablet, 2-drug combination. In contrast to DTG plus RPV, DTG plus 3TC has proven effective as initial therapy in treatment-naive individuals. GEMINI-1 and GEMINI-2 were identically designed large phase 3 studies comparing the 2-drug regimen of DTG plus 3TC with the 3-drug regimen DTG plus FTC/TDF in ART-naive participants. At week 48 the 2-drug regimen was found to be noninferior to the 3-drug regimen, with fewer adverse events. DTG/3TC was not associated with emergence of treatment-associated mutations.21 Multiple switch studies have demonstrated maintenance of viral suppression when patients with virological control are transitioned to DTG plus 3TC, including a large phase 3 switch study called TANGO that is still ongoing .22-25
For now, DTG/3TC is only recommended in HHS guidelines for use in patients for whom ABC, TDF, or TAF is contraindicated or not optimal. This includes patients who have cardiovascular disease (ABC), chronic kidney disease (TDF, TAF), or osteoporosis (TDF, TAF).17 However, based on the promising data from the GEMINI studies, this 2-drug strategy is a step toward decreased cumulative drug exposure for people living with HIV.
Juluca Combination DTG/RPV (Juluca, ViiV Healthcare), approved in November 2017, was the first complete single-tablet regimen consisting of only 2 drugs. The combination has proven effective as a switch regimen in treatment-experienced patients who are virologically suppressed. In 2 identical phase 3, open-label trials, SWORD-1 and SWORD-2, treatment-experienced participants without NNRTI resistance and with good virological control were randomly assigned to switch to DTG plus RPV or remain on their current ART. Participants who were switched to DTG plus RPV maintained virological suppression at 48 weeks, and experienced fewer adverse events with better renal and bone outcomes than participants who remained on their current ART.18 Multiple observational studies have confirmed that patients maintain durable virological suppression after switching to DTG plus RPV.19,20
Coformulated DTG/RPV is a promising NRTI-sparing regimen for patients with side effects to current ART, contraindications to NRTIs, or who are just looking for regimen simplification. It is a particularly good option for patients with renal impairment, as exposure to DTG and RPV is not affected by decreased renal function. This combination has only been studied as a switch regimen in patients who are already virologically suppressed with no history of treatment failure or major drug resistance, and has not been assessed in treatment-naive individuals.
What drugs in the pipeline hold the most promise?
Injectable Agents
Albuvirtide Albuvirtide (ABT), which is being developed by Frontier Biotechnologies, is a novel fusion inhibitor that binds to the gp41 envelope protein, preventing HIV from fusing with the host cell membrane and entering the cell. Like the other medication in this class, enfuvirtide (Fuzeon, Genentech), it is administered via subcutaneous injection, but due to its prolonged half-life, it can be given just once weekly. Currently, the target population for this medication is individuals with multiclass resistance or intolerance of multiple classes of medications. The TALENT study is a phase 3 trial comparing ritonavir-boosted lopinavir plus either ABT or 2 NRTIs in treatment-experienced participants in China experiencing virological failure. An interim analysis at week 48 found that 80% of patients in the ABT arm had a viral load less than 50 copies/mL compared with 66% in the NRTI arm. These results were presented at a conference in 201626 and a manuscript available online in preprint format,27 but has not yet been published in a peer-reviewed journal. Albuvirtide is only approved for second-line therapy in China where access to alternative oral medications, like the INSTIs, may be more limited.
Maintenance therapy for individuals with good virological control is another potential use for ABT when combined with other agents. There is an ongoing phase 2 study of combination ABT plus 3BNC117 (a broadly neutralizing antibody) as long-acting maintenance therapy in virologically suppressed patients.28
Oral Agents
Fostemsavir Fostemsavir (FTR), the prodrug of temsavir being developed by ViiV Healthcare, is a first-in-class gp120 attachment inhibitor that works by blocking viral attachment to the host CD4 cell. In contrast with maraviroc (Selzentry, ViiV Healthcare), a CCR5 attachment inhibitor, it is active regardless of viral tropism, and there is no cross-resistance to any existing ART.29 The BRIGHTE study is an ongoing phase 3 trial of patients experiencing virological failure with multiclass resistance and no full viable regimens available. When FTR was added to an optimized backbone for patients with 1 or 2 remaining active ART classes, 54% of individuals achieved a viral load less than 40 copies/mL and 77% achieved a viral load less than 400 copies/mL at 24 weeks. For patients who had 0 remaining active ART classes, 36% achieved a viral load less than 40 copies/mL at 24 weeks.30 Rates of virological suppression were maintained through 48 weeks, although there was significant attrition from the trial.31,32 In contrast to IBA and ABT, FTR is taken orally and may be preferred salvage therapy for patients with multiclass resistance. Patients with resistance to all existing classes of medications may require a combination of these new agents to achieve viral suppression.
Islatravir Islatravir (ISL, formerly MK-8591), a nucleoside reverse transcriptase translocation inhibitor being developed by Merck, is an adenosine analog that acts as a reverse transcriptase chain terminator and prevents DNA translocation.33 The DRIVE2Simplify study is an ongoing phase 2 clinical trial of daily administration of ISL with DOR as a 2-drug strategy. If outcomes are favorable, this could add to the growing menu of 2-drug regimens.34
A key feature of the medication is that due to a prolonged half-life, it holds potential for once-weekly dosing, which may be an appealing strategy to decrease pill burden. Moreover, pharmacokinetic studies show that therapeutic levels of ISL can be sustained for longer than 6 months when the drug is administered via long-term drug-eluting implants.35 The implants hold promise for both HIV pre-exposure prophylaxis (PrEP) when used as monotherapy, and as treatment when used with other medications.
Long-Acting Injectable Combination
Cabotegravir Plus Rilpivirine Cabotegravir (CAB), a novel long-acting injectable INSTI with a structure and resistance profile similar to DTG, is being developed by ViiV Healthcare. It is combined with injectable RPV (Edurant, Janssen Therapeutics) and is likely to be the first approved long-acting injectable ART regimen. In the ATLAS trial, treatment-experienced patients with virological control and no baseline NNRTI or INSTI mutations who switched to monthly injections of CAB and RPV (each drug must be administered in separate injections) had similar virological outcomes compared with those who continued on 3-drug oral ART.36 In the FLAIR trial of treatment-naive participants who received a 20-week lead-in period of oral DTG/ABC/3TC, switching to monthly injections of CAB and RPV was noninferior to continuation of DTG/ABC/3TC.37 The most common adverse events were injection site reactions that were relatively mild (grade 1 or 2). In a subjective assessment of patient satisfaction with injectable formulations, many patients were found to prefer monthly injections over daily oral medications.38 Long-acting injectable regimens may prove to be a paradigm shift for patients who struggle with daily adherence to oral medications, or who simply prefer monthly injections over taking pills every day.
Long-acting injectable CAB also is being evaluated for PrEP in HIV-uninfected individuals.39
Conclusion
In 2019, most patients with HIV who are engaged in care are virologically suppressed, but recent advances have led to simplified regimens, decreased cumulative drug exposure, and expanded therapeutic options for patients with multiclass resistance. The field continues to evolve as new drug classes, long-acting injectables, and drug-eluting implants appear on the horizon.
References
- Molina JM, Squires K, Sax PE, et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naïve adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV. 2018;5(5):e211-e220.
- Molina JM, Squires K, Sax PE, et al. Doravirine (DOR) versus ritonavir-boosted darunavir (DRV+r): 96-week results of the randomized, double-blind, phase 3 DRIVE-FORWARD noninferiority trial. Presented at: 22nd International AIDS Conference; July 23-27, 2018; Amsterdam, the Netherlands. Abstract LBPEB017.
- Orkin C, Squires KE, Molina JM, et al. Doravirine/lamivudine/tenofovir disoproxil fumarate is non-inferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naïve adults with human immunodeficiency virus-1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis. 2019;68(4):535-544.
- Johnson M, Kumar P, Molina JM, et al. Switching to doravirine/lamivudine/tenofovir disoproxil fumarate (DOR/3TC/TDF) maintains HIV-1 virologic suppression through 48 weeks: results of the DRIVE-SHIFT trial. J Acquir Immune Defic Syndr. 2019;81(4):463-472.
- Feng M, Wang D, Grobler JA, et al. In vitro resistance selection with doravirine (MK-1439), a novel nonnucleoside reverse transcriptase inhibitor with distinct mutation development pathways. Antimicrob Agents Chemother. 2015;59(1):590-598.
- Thompson M, Orkin C, Molina JM, et al. Once-daily doravirine for initial treatment of adults living with HIV-1: an integrated safety analysis. Clin Infect Dis. 2019 May 23. (Epub ahead of print). doi: 10.1093/cid/ciz423
- Emu B, Fessel J, Schrader S, et al. Phase 3 study of ibalizumab for multidrug-resistant HIV-1. N Engl J Med. 2018;379(7):645-654.
- Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390(10107):2073-2082.
- Wohl DA, Yazdanpanah Y, Baumgarten A, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e355-e363.
- Molina JM, Ward D, Brar I, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e357-e365.
- Daar ES, DeJesus E, Ruane P, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e347-e356.
- Oliveira M, Ibanescu RI, Anstett K, et al. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology. 2018;15(1):56.
- Apostolova N, Funes HA, Blas-Garcia A, et al. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother. 2015;70(10):2693-2708.
- ENCORE1 Study Group; Carey D, Puls R, Amin J, et al. Efficacy and safety of efavirenz 400 mg daily versus 600 mg daily: 96-week data from the randomised, double-blind, placebo-controlled, non-inferiority ENCORE1 study. Lancet Infect Dis. 2015;15(7):793-802.
- Eron JJ, Orkin C, Gallant J, et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2018;32(11):1431-1442.
- Orkin C, Molina JM, Negredo E et al. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV. 2018;5(1):e23-e34.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services. www.aidsinfo.nih.gov/?ContentFiles/?AdultandAdolescentGL.pdf. Accessed September 4, 2019.
- Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391(10123):839-849.
- Capetti AF, Cossu MV, Sterrantino G, et al. Dolutegravir plus rilpivirine as a switch option in cART-experienced patients: 96-week data. Ann Pharmacother. 2018;52(8):740-746.
- Palacios R, Mayorga M, González-Domenech CM, et al. Safety and efficacy of dolutegravir plus rilpivirine in treatment-experienced HIV-infected patients: the DORIVIR study. J Int Assoc Provid AIDS Care. 2018;17:2325958218760847.
- Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naïve adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393(10167):143-155.
- Joly V, Burdet C, Landman R, et al. Dolutegravir and lamivudine maintenance therapy in HIV-1 virologically suppressed patients: results of the ANRS 167 trial (LAMIDOL). J Antimicrob Chemother. 2019;74(3):739-745.
- Li JZ, Sax PE, Marconi VC, et al. No significant changes to residual viremia after switch to dolutegravir and lamivudine in a randomized trial. Open Forum Infect Dis. 2019;6(3):ofz056.
- Taiwo BO, Marconi VC, Berzins B, et al. Dolutegravir plus lamivudine maintains human immunodeficiency virus-1 suppression through week 48 in a pilot randomized trial. Clin Infect Dis. 2018;66(11):1794-1797.
- Van Wyk J, Ajana F, Bisshop F, et al. Switching to DTG+3TC is non-inferior to continuing a TAF-based regimen (TBR) in maintaining virologic suppression through 24 weeks (TANGO study). Presented at 2019 International AIDS Society, July 21-24, 2019; Mexico City, Mexico. Abstract WEAB0403LB.
- Wu H, Yao C, Su B, et al. Efficacy and safety of long-acting HIV fusion inhibitor albuvirtide in antiretroviral-experienced adults with HIV-1: interim 48-week results from the randomised, controlled, phase 3, non-inferiority TALENT study. Presented at: 2016 International Congress on Drug Therapy in HIV Infection; October 23-26, 2016; Glasgow, United Kingdom. Oral abstract O336.
- Wu H, Yao C, Su B, et al. Efficacy and safety of long acting HIV fusion inhibitor albuvirtide in antiretroviral-experienced adults with HIV-1: interim 48 week results from the randomized, controlled, phase 3 trial, non-inferiority TALENT study. Lancet 2019 Jan 8. (Epub ahead of print).
- ClinicalTrials.gov. Albuvirtide and 3BNC117 as long-acting maintenance therapy in virologically suppressed subjects (ABL). clinicaltrials.gov/ct2/show/NCT03719664. Accessed September 4, 2019.
- Nowicka-Sans B, Gong YF, McAuliffe B, et al. In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob Agents Chemother. 2012;56(7):3498-3507.
- Kozal M, Aberg J, Pialoux G, et al. Phase 3 study of fostemsavir in heavily treatment-experienced HIV-1-Infected participants: day 8 and week 24 primary efficacy and safety results (BRIGHTE Study, Formerly 205888/AI438-047). Presented at: 16th European AIDS Conference; October 25-27, 2017; Milan Italy.
- Molina JM, Aberg J, Cassetti I, et al. Phase 3 study of fostemsavir in heavily treatment-experienced O334B HIV—infected participants: BRIGHTE week 48 subgroup analysis in randomised cohort participants. Presented at: 2018 International Congress on Drug Therapy in HIV Infection; October 28-31, 2018; Glasgow, United Kingdom. Oral abstract O344A.
- Aberg, J, Molina JM, Kozal M, et al. Week 48 Safety and Efficacy of the HIV-1 attachment inhibitor prodrug fostemsavir in heavily treatment-experienced participants (BRIGHTE Study). Presented at: 2018 International Congress on Drug Therapy in HIV Infection; October 28-31, 2018; Glasgow, United Kingdom. Oral abstract O344A.
- Michailidis E, Huber AD, Ryan EM, et al. 4’-Ethynyl-2-fluoro-2’-deoxyadenosine (EFdA) inhibits HIV-1 reverse transcriptase with multiple mechanisms. J Biol Chem. 2014;289(35):24533-24548.
- ClinicalTrials.gov. MK-8591 with doravirine and lamivudine in participants infected with human immunodeficiency virus type 1 (MK-8591-011). clinicaltrials.gov/ct2/show/NCT03272347. Accessed July 15, 2019.
- Barrett SE, Teller RS, Forster SP, et al. Extended-duration MK-8591-eluting implant as a candidate for HIV treatment and prevention. Antimicrob Agents Chemother. 2018;62(10). doi:10.1128/AAC.01058-18
- Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir + rilpivirine as maintenance therapy: ATLAS week 48 results. Presented at: Conference on Retroviruses and Opportunistic Infections 2019; March 4-7, 2019; Seattle, WA. Abstract 139.
- Orkin C, Arasteh K, Hernandez-Mora MG, et al. Long-acting cabotegravir + rilpivirine for HIV maintenance: FLAIR week 48 results. Presented at: Conference on Retroviruses and Opportunistic Infections 2019; March 4-7, 2019; Seattle, WA. Abstract 140LB.
- Murray MI, Markowitz M, Frank I, et al. Satisfaction and acceptability of cabotegravir long-acting injectable suspension for prevention of HIV: patient perspectives from the ECLAIR trial. HIV Clin Trials. 2018;19(4):129-138.
- Stellbrink HJ, Hoffmann C. Cabotegravir: its potential for antiretroviral therapy and preexposure prophylaxis. Curr Opin HIV AIDS. 2018;13(4):334-340.
Copyright © 2019 McMahon Publishing, 545 West 45th Street, New York, NY 10036. Printed in the USA. All rights reserved, including the right of reproduction, in whole or in part, in any form.

Download to read this article in PDF document:![]() Advances in HIV Therapy: What Is New and What Comes Next?
Advances in HIV Therapy: What Is New and What Comes Next?