Although pivmecillinam has been available for more than 40 years in Canada and Europe, it is a new product in the United States, and it looks like pivmecillinam (Pivya, Utility Therapeutics) will be a valuable first-line treatment for uncomplicated urinary tract infections (UTIs) with no clear evidence of emerging resistance (Open Forum Infect Dis 2024;11[6]:ofae296 doi:10.1093/ofid/ofae296).
“This is a drug that has been out there and has been used frequently in Europe with quite good results,” said Keith S. Kaye, MD, MPH, the chief of the Division of Allergy, Immunology and Infectious Diseases, Rutgers Health Robert Wood Johnson Medical School, in New Brunswick, N.J., and a co-author of the study.
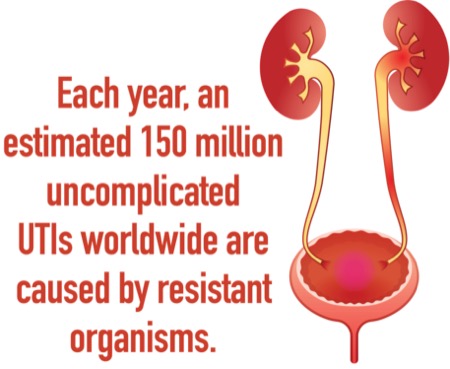
Antimicrobial resistance to common uropathogens that cause uncomplicated UTIs are a growing international problem: An estimated 150 million such infections occur worldwide each year. In the United States alone, the societal costs of these infections is approximately $3.5 billion. An increasing proportion of these infections are caused by antibiotic-resistant gram-negative bacteria such as Escherichia coli, as well as extended-spectrum beta-lactamase–producing (ESBL) Enterobacterales.
Despite its international track record, pivmecillinam, an oral prodrug of mecillinam, only was approved by the FDA in 2024 for treatment of uncomplicated UTIs. Prior research has shown microbiological response rates for uncomplicated UTIs between 75% and 94%, with clinical response rates ranging from 82% to 95%.
“It’s been a drug that a lot of us have looked at since [it was added to] the IDSA [Infectious Diseases Society of America] UTI guidelines more than a decade ago,” said James S. Lewis II, PharmD, FIDSA, the co-director of antibiotic stewardship at Oregon Health & Science University, in Portland, and a member of the Infectious Disease Special Edition editorial advisory board. “That was when the drug first got my attention, but it has stayed prevalent in the infectious disease literature during the last decade in Europe.”
In the Forum review, the authors examined 38 studies that investigated the susceptibility surveillance data and evidence of the susceptibility of common uncomplicated UTI–causing uropathogens to mecillinam. They found no clear evidence to suggest that resistance to mecillinam among common uropathogens has increased.
The review found 22 studies reporting data about the overall susceptibility of mecillinam to E. coli. In most of those studies, the susceptibility exceeded 95%. Only four reported a susceptibility rate below 90%, but no studies showed a rate less than 80%.
The authors also analyzed consumption data from Denmark and found that resistance rates significantly decreased despite increased consumption of the drug.
“I think it is encouraging,” Dr. Kaye said. “It’s quite remarkable, actually. When you look at the rates on increasing quinolone and trimethoprim-sulfamethoxazole resistance in E. coli, the fact that resistance has not emerged to pivmecillinam to any notable degree is quite impressive.”
One of the most recent and largest studies involved more than 1.5 million isolates from 2010 to 2020 in Denmark, Norway and Sweden. Susceptibility to mecillinam remained stable during that time, with rates between 94% and 96%.
Among the 15 studies that reported data about resistant phenotypes of E. coli excluding carbapenem-resistant strains—mainly ESBL-producing E. coli—most reported susceptibility rates above 90%, and none reported rates less than 82.5%.
For carbapenem-resistant or carbapenemase-producing Klebsiella pneumoniae, reported susceptibility rates varied from 8.5% to 66.9%.
“I’m excited about it,” said Dr. Lewis, who was not involved in the study. “It gives us an oral option for ESBL-producing E. coli in urinary tracts, it comes with a ton of safety data, and it’s a penicillin-class drug. There’s a lot to like there.”
Overall, the review authors noted that their findings demonstrate a good level of activity against E. coli in uncomplicated UTIs, noting that susceptibility of mecillinam to E. coli exceeded 83% in 21 published studies, 90% in 17 studies and 95% in 15 studies.
“I think the overall published volume of clinical experience over decades is impressive, particularly when you review it from start to finish,” Dr. Kaye explained. “This is an ‘old’ antibiotic that is ‘new’ to the United States. It has been used with excellent clinical success in Europe for years, it has a good safety record, and it has good activity against the most common cause of uncomplicated UTI, E. coli.”
Regarding the drug’s future in the U.S. market, Dr. Lewis is optimistic.
“The susceptibility testing is already in place, the break points are already in place, there’s an extensive safety history and you already have an IDSA guideline for it,” he said. “That’s not common for most new drugs in the U.S. market.”
The sources reported no relevant financial disclosures.
This article is from the February 2025 print issue.