The treatment of drug-susceptible and -resistant tuberculosis among children and adults should focus on the use of all-oral, shorter treatment regimens, according to updated clinical practice guidelines, which were developed by an expert panel featuring representatives from the American Thoracic Society, CDC, European Respiratory Society and Infectious Diseases Society of America (Am J Resp Crit Care Med 2025;211[1]:15-33).
“People with both drug-susceptible and drug-resistant TB and their clinicians would prefer shorter treatment duration, oral regimens, reduced number of medications, reduced pill burden, and less adverse drug effects from TB treatment,” said joint first author of the report, Sonal S. Munsiff, MD, an infectious disease physician and a clinical trialist at the University of Rochester, in New York. “There has been a concerted effort to develop shorter treatments for TB after decades of little drug development.”
For most patients with drug-susceptible and drug-resistant TB, treatment regimens have been significantly reduced, and now last between four and six months.
“The guideline offers information on who is eligible for these new regimens, drug dosages and monitoring recommendations for using these regimens,” Dr. Munsiff said.
For adolescents and adults with drug-susceptible pulmonary TB, the panel recommends two months of isoniazid, moxifloxacin, pyrazinamide and rifapentine, followed by two months of isoniazid, moxifloxacin and rifapentine.
Most children and adolescents with non-severe TB can be treated in four months instead of six, with a regimen consisting of two months of ethambutol, isoniazid, pyrazinamide and rifampin, followed by two months of isoniazid and rifampin.
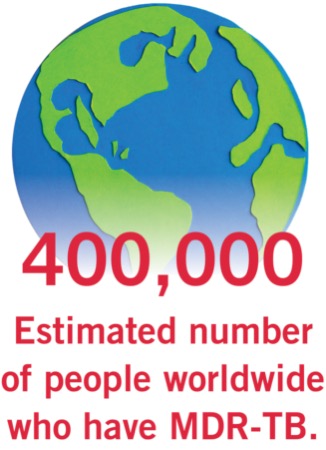
People with multidrug- and rifampin-resistant TB disease can be treated in six months, significantly less than the previously recommended 15 to 24 months. For those older than 14 years of age with rifampin-resistant, fluoroquinolone-susceptible TB, this consists of six months of bedaquiline, linezolid, moxifloxacin and pretomanid. In those older than 14 with rifampin-resistant TB and fluoroquinolone resistance or intolerance, treatment can consist of six months of bedaquiline, linezolid and pretomanid.
“For drug-susceptible TB, we now have effective regimens that reduce treatment duration by one-third,” Dr. Munsiff added. “For drug-resistant TB, we now have six months for all-oral regimens that reduce treatment duration by about two-thirds, compared with the old standard-of-care regimens. These regimens can also be used for people with intolerance to rifampin, which is a key drug for short course chemotherapy of tuberculosis.”
Shorter and more effective treatment courses may improve adherence, reduce adverse events and the time someone is ill, as well as hasten other aspects of recovery, according to Dr. Munsiff.
Additionally, all-oral regimens may mean easier administration, particularly if the number of pills can be reduced, and can help reduce strain on healthcare professionals involved with directly observed therapy (DOT). “A shorter [course] also reduces the duration of DOT, which is personnel-intensive,” Dr. Munsiff said.
While the recommendations reflect the current status of study regarding TB therapies, future research will aim to address lingering questions. Many of the current studies are small, and may not accurately reflect populations of those with TB in the United States and Europe. Additionally, the studies haven’t included all patient populations and forms of TB.
“We urgently need studies of treatment with these regimens of individuals excluded from these trials so that these vulnerable populations can benefit from these regimens,” Dr. Munsiff said. “We also need studies about implementation challenges, access to rapid diagnostics for susceptibility to the new drugs, emergence of resistance to the new drugs and other issues which will inform future guidelines.”
Dr. Munsiff also stressed the importance of continued TB monitoring.
“Monitoring and evaluation are also crucial to ensure that the guidelines are being followed correctly and to assess their impact on TB control,” Dr. Munsiff said. “This will help identify any challenges or barriers to implementation and allow for adjustments to be made to improve the effectiveness of TB care.”
While those questions are being addressed, focus will fall on sharing the new recommendations and educating healthcare professionals regarding their use.
“The next steps include widespread dissemination of the guidelines to healthcare providers, offering training on the new regimens and ensuring that the necessary medications are available and affordable,” Dr. Munsiff said.
Dr. Munsiff reported no relevant financial disclosures.
This article is from the February 2025 print issue.