Earlier detection of HIV infection along with more effective highly active antiretroviral therapy (ART) has markedly improved the life expectancy of HIV-positive (HIV+) patients in the United States. According to a study by Siddiqi et al, from 2008 to 2011, the average life expectancy after receiving an HIV diagnosis increased by roughly 3 years, with improvements noted across all races and ethnicities and in all modes of transmission. Interestingly, a disproportionate increase in the life expectancy of men with HIV compared with women was observed in this study.1
Samji et al reported that 20-year-old HIV+ patients on ART living in the United States and Canada are expected to survive into their early 70s, which is approaching estimates for the general population of the same age. However, it is important to note that this projection is based on HIV+ patients who are newly initiating ART and do not necessarily represent life expectancy for those who have had their HIV diagnosis for a longer period.2 In 2015, only 39% of HIV+ patients in the United States were aged 50 years or older, while by 2035, 74% will fall into this age bracket; 44% of these HIV+ patients will have 3 or more chronic diseases relate d to aging compared with 2015 when most HIV+ patients had either 1 or none. Moderate cardiovascular disease (CVD), defined as hypertension and/or dyslipidemia, also will comprise a large portion of this burden, with a predicted increase from 61% of HIV+ patients on ART in 2015 to 84% in 2035. The increased burden of such noninfectious chronic diseases will drive up treatment costs, accounting for 55.7% of the total HIV care expenditure by 2035.3
In view of these trends, understanding the care needs of HIV+ patients with CVD is of the utmost importance. This review serves as an update to information presented in the Spring 2018 IDSE edition and will examine the factors that influence cardiovascular health in HIV+ patients, including ART and HIV infection itself, and also assesses current and potential future management practices.
Epidemiology of CVD
Although the HIV+ population remains afflicted by AIDS-related deaths, the proportion of CVD mortality increased more than 2-fold from 1999 to 2013. When examining the categories of CVD, the HIV+ population experienced a 3-fold increase in ischemic heart disease deaths, whereas the general population saw a decrease. Furthermore, these changes occurred in the setting of a reduction in overall mortality in the HIV+ population. HIV+ patients were more likely to die of hypertensive heart, renal, and pulmonary circulatory diseases, whereas the general population tended to have deaths from heart failure (HF) and/or cardiomyopathy or cerebrovascular disease.4
Additionally, CVD in HIV+ women is more common than in women without HIV.6 Regarding CVD-related death, HIV+ patients typically experience this later in the disease process but tend to present at younger ages than the general population.4
Similar to the general population, traditional risk factors for CVD contribute to the overall poor cardiac health profile of the HIV+ population. Levy et al reported the prevalence of various comorbidities in the HIV+ population as follows: hypertension, 50%; dyslipidemia, 48%; multiple conditions, 46%; obesity, 35%; and type 2 diabetes mellitus, 13%. The longer an individual has HIV, the higher the probability of having type 2 diabetes and dyslipidemia, and longer duration of ART is associated with a higher metabolic risk. HIV+ men are more likely to have dyslipidemia and HIV+ women are more likely to have type 2 diabetes and obesity.9
The HIV+ population has changed from once being affected by physiologic wasting to one affected by obesity, which has led to complications from numerous metabolic comorbidities. From 1998 to 2010, the median body mass index (BMI) increased from 23.8 kg/m2 to 24.8 kg/m2 and the percentage of obese individuals increased from 9% to 18% in HIV+ patients at initiation of ART. After ART initiation, weight gain commonly occurs, leading to an increased prevalence of obesity. Smoking is also a risk factor and is associated with a 2-fold increase in mortality among HIV+ tobacco smokers compared with nonsmokers. This excess mortality in HIV+ smokers is mainly due to non-AIDS cancers and CVD. Comparing 35-year-old HIV+ male smokers and same-aged HIV+ male nonsmokers, smokers have been found to lose 7.9 life-years.12 Age and smoking also are associated with carotid intima-media thickness (CIMT), a measure of subclinical atherosclerosis. Older HIV+ smokers have a greater CIMT than older HIV-negative smokers. This finding suggests that HIV infection changes how age and smoking interact with CIMT.13
People who are HIV+ also engage in less physical activity, an important component of cardiovascular health. They have less ventilatory efficiency, an indicator of worse cardiovascular fitness, than HIV-negative individuals. Webel et al demonstrated associations between higher step counts (a measure of physical activity) and reduced insulin resistance and improved oxygen uptake in physically active HIV+ patients. Thus, a key aspect of managing HIV+ patients, especially those with greater CVD risk, is promotion of physical activity.14
People with HIV have an increased risk for developing focal carotid artery plaques compared with HIV-negative individuals, even if they are virologically suppressed. The risk for plaque formation associated with HIV infection is greater than that associated with smoking.15 In a study by O’Dwyer et al, HIV+ men with acute coronary syndrome were found to have a lower plaque burden than matched controls. The presence of a lower plaque burden resulting in acute coronary syndrome suggests the presence of vulnerable or high-risk plaques and emphasizes the danger of subclinical atherosclerosis in HIV+ patients.17
HIV-Related Risk Factors
The typical target of HIV is CD4+ lymphocytes, but studies showed that the virus also targets macrophages, monocytes, and endothelial cells. Macrophages infected with HIV-1 exhibit an impaired cholesterol efflux; this leads to elevated levels of intracellular cholesterol, thus triggering necrosis or apoptosis and potentially causing plaques.18 Khera et al showed that patients with greater efflux capacity tended to have lower levels of coronary disease, and greater efflux was found to protect macrophages from apoptosis.19
This proatherogenic phenotype of monocytes persists even in HIV+ patients who are virologically suppressed and may contribute mechanistically to increased atherosclerosis in this population.20 There is also a correlation between HIV infection and increased left ventricular dysfunction leading to HF. Higher left ventricular mass index associated with lower CD4+ levels compared with controls and increased diastolic dysfunction in the HIV+ patient population suggest the role of immunodeficiency in HF risk and not that of traditional risk factors.21
A study in Nigeria recruited 150 age- and sex-matched controls and found echocardiogram abnormalities in 55.3% of the HIV+ patients compared with 2.7% of controls (P<0.001). All the structural dimensions of the cardiac chambers were significantly greater than those in controls except for left atrial dimension (LAD). When the patients were stratified into 2 groups with a CD4 count less than or more than 200 cells/mm3, the structural chamber dimensions were similar in both groups. However, this did not seem to be related to HIV disease severity because the chamber dimensions were similar in patients regardless of whether they had less than or more than 200 cells/mm3.22 The role of echocardiography is still unclear and needs to be better defined, and additional studies are needed to determine the role of HIV in CAD pathogenesis.
ART-Related Risk
ART has contributed to the increase in life expectancy among HIV+ patients; however, it also has increased the risk for CVD. One of the main concerns with ART involves serum lipid levels and dyslipidemia. Among HIV+ patients, those exposed to ART have higher mean total cholesterol levels and higher mean low-density lipoprotein (LDL) cholesterol levels than unexposed HIV+ patients; the former is especially associated with protease inhibitor (PI)-based regimens. ART regimens also result in higher rates of hypercholesterolemia and hypertriglyceridemia. Of HIV+ patients on PIs, 27.2% have hypercholesterolemia compared with 9.3% of unexposed HIV+ patients. Similarly, 37.1% of treated HIV+ patients have hypertriglyceridemia versus 17.7% of unexposed HIV+ patients; levels of both hypercholesterolemia and hypertriglyceridemia increase progressively with duration of ART.23
In addition to dyslipidemia, HIV+ patients on ART are susceptible to increases in BMI. Treatment duration of 3 years or more is associated with a BMI increase regardless of ART.27
In a study by Drojee et al, use of abacavir was associated with an increased risk for CVD events. Whereas the incidence of CVD events related to current abacavir exposure was 9.74 per 1,000 person-years, the rate in relation to other current ART use was 5.75 per 1,000 person-years. Risk for CVD events remained elevated even after 6 months of stopping abacavir use compared with no exposure to abacavir.29 Maraviroc, a CCR5 antagonist, was evaluated for whether it could modulate the atherosclerotic burden in aviremic PI-treated HIV+ patients who underwent maraviroc intensification. Intensification (n=6) resulted in a significant reduction in intima-media thickness, pulse wave velocity, and triglycerides compared with baseline. It was also associated with a significant reduction in interleukin (IL)-6 microbial translocation indexes and improvement in endothelial function. These changes support the protective role of CCR5 antagonists on atherosclerotic burden.30
In the NEAT 022 study, 415 virologically suppressed patients with high CVD risk (age >50 years and/or Framingham risk score >10% at 10 years) were randomly assigned to either continue a regimen of a boosted PI plus 2 nucleoside reverse transcriptase inhibitors (NRTIs) or switch to dolutegravir (DTG) plus 2 NRTIs. The 48-week data showed similar virologic suppression on both regimens, with more than 93% overall suppression. Dramatic improvements in lipid parameters (including total cholesterol, non–high-density lipoprotein [HDL] and LDL cholesterol, triglycerides, and total cholesterol-to-HDL ratio) were observed in patients switching to DTG-based ART; however, HDL remained largely unchanged in either group. These data confirm that a switch from a boosted PI–based ART to a DTG-based regimen may improve lipid parameters and possibly reduce CVD risk.31
Drug–Drug Interactions
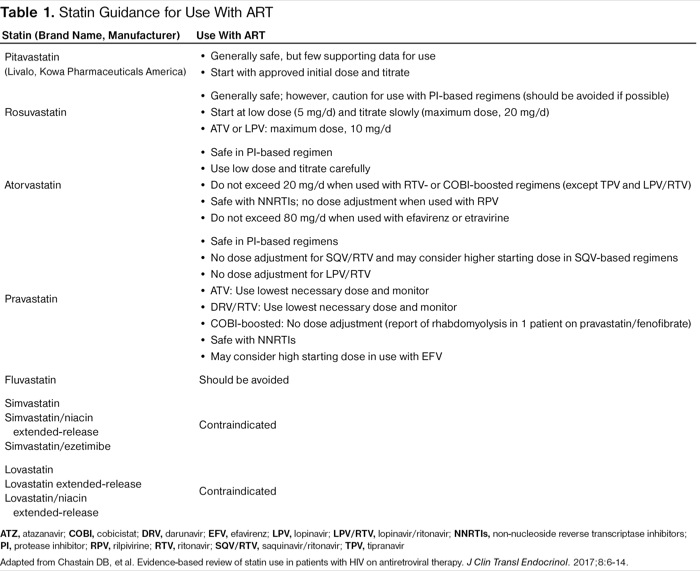
The underuse of cardiovascular medications in the HIV+ population may be due in part to concern about drug interactions with ART (Tables 1 and 2). Molas et al described the potential drug–drug interactions in HIV+ patients on ART and showed the importance of monitoring patients to ensure safety. In the study population, 70% of ART-treated HIV+ patients were receiving comedications with the most common category being cardiovascular drugs. Evaluating for potential drug–drug interactions via an online system, the investigators found that 44.7% of the study population had at least 1 clinically significant potential drug–drug interaction, with the most interactions associated with ritonavir-boosted PIs. Ritonavir-boosted PIs were a risk factor for potential drug–drug interactions, as were non-NRTIs and taking 2 or more comedications. A management plan of changing comedications or the ART regimen was recommended for 23.6% of HIV+ patients with comedications and potential drug–drug interactions.34
| Table 1. Statin Guidance for Use With ART | |
| Statin (Brand Name, Manufacturer) | Use With ART |
|---|---|
| Pitavastatin (Livalo, Kowa Pharmaceuticals America) |
|
| Rosuvastatin |
|
| Atorvastatin |
|
| Pravastatin |
|
| Fluvastatin | Should be avoided |
| Simvastatin Simvastatin/niacin extended-release Simvastatin/ezetimibe | Contraindicated |
| Lovastatin Lovastatin extended-release Lovastatin/niacin extended-release | Contraindicated |
| ATZ, atazanavir; COBI, cobicistat; DRV, darunavir; EFV, efavirenz; LPV, lopinavir; LPV/RTV, lopinavir/ritonavir; NNRTIs, non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitor; RPV, rilpivirine; RTV, ritonavir; SQV/RTV, saquinavir/ritonavir; TPV, tipranavir Adapted from Chastain DB, et al. Evidence-based review of statin use in patients with HIV on antiretroviral therapy. J Clin Transl Endocrinol. 2017;8:6-14. | |
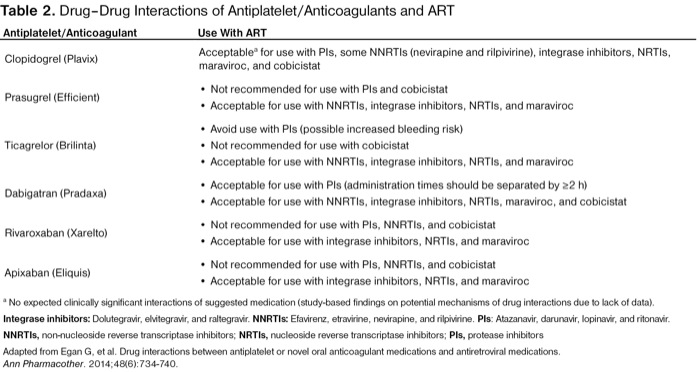
| Table 2. Drug–Drug Interactions of Antiplatelet/Anticoagulants and ART | |
| Antiplatelet/Anticoagulant | Use With ART |
|---|---|
| Clopidogrel (Plavix) | Acceptablea for use with PIs, some NNRTIs (nevirapine and rilpivirine), integrase inhibitors, NRTIs, maraviroc, and cobicistat |
| Prasugrel (Efficient) |
|
| Ticagrelor (Brilinta) |
|
| Dabigatran (Pradaxa) |
|
| Rivaroxaban (Xarelto) |
|
| Apixaban (Eliquis) |
|
a No expected clinically significant interactions of suggested medication (study-based findings on potential mechanisms of drug interactions due to lack of data). Integrase inhibitors: Dolutegravir, elvitegravir, and raltegravir. NNRTIs: Efavirenz, etravirine, nevirapine, and rilpivirine. PIs: Atazanavir, darunavir, lopinavir, and ritonavir. NNRTIs, non-nucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors Adapted from Egan G, et al. Drug interactions between antiplatelet or novel oral anticoagulant medications and antiretroviral medications. Ann Pharmacother. 2014;48(6):734-740. | |
In addition, McNicholl et al studied polypharmacy in older HIV+ patients to evaluate potentially inappropriate prescribing. Cardiovascular conditions, such as hypertension, dyslipidemia, and CAD, contributed to the list of common comorbidities in this population. In 20 HIV+ patients, a total of 25 contraindicated drug interactions were discovered, with 11.5% involving cardiovascular medications. Most of the contraindicated drug interactions associated with ART were due to PI use.35
Prevention and Screening
To better manage CVD risk in HIV+ patients, emphasis must be placed on both efficient and cost-effective prevention and screening strategies.
Most screening guidelines in place for determining CVD risk were developed for use in the general population and not necessarily HIV+ patients. Phan et al assessed the value of the 2013 American College of Cardiology/American Heart Association (ACC/AHA) and 2004 Adult Treatment Panel III (ATP III) guidelines for statin therapy when applied to the HIV+ population. In their overall study population, the ACC/AHA recommended 26.4% of HIV+ patients for statin therapy, whereas the ATP III recommended only 13.6%. These patients tended to have more comorbidities and traditional cardiovascular risk factors. In HIV+ patients with carotid plaque, both guidelines did not recommend statin therapy for roughly 2 out of 3 HIV+ patients. Both guidelines were associated with baseline CIMT and CIMT progression but did not serve as predictors of death.39
A multitude of CVD risk estimators is available for use, but there is no consensus on which one is the best for the HIV+ population. Krikke et al compared the effectiveness of the Framingham Heart Study general CVD risk score (FRS), Framingham Heart Study coronary heart disease risk score (FHS-CHD), atherosclerotic cardiovascular disease risk score (ASCVD), Systematic COronary Risk Evaluation for the Netherlands (SCORE-NL), and Data Collection on Adverse Events of Anti-HIV Drugs risk score (D:A:D). Of note, the D:A:D model is the only HIV-specific risk score. In terms of overall cumulative risk, the D:A:D, ASCVD, and SCORE-NL estimated similar risk distributions whereas the FRS and FHS-CHD both estimated a higher overall cumulative risk. Across all risk prediction models, most HIV+ patients were categorized as low/medium risk. When comparing general risk scores with the HIV-specific D:A:D risk score, ASCVD and SCORE-NL produced similar results, but both the FRS and FHS-CHD placed more patients into a higher risk category (ie, overestimated cardiovascular risk).40
Thompson-Paul et al compared the abilities of the FRS, ACC/AHA pooled cohort equation (PCE), D:A:D, and SCORE risk prediction models. The FRS, PCE, and D:A:D were all able to adequately distinguish high-risk HIV+ patients from low-risk HIV+ patients, but PCE and D:A:D estimated a lower CVD event risk. In contrast, SCORE was unable to appropriately categorize high- and low-risk HIV+ patients.41
Raggi et al specifically assessed cardiovascular event prediction and reported that PCE and D:A:D were better at categorizing HIV+ patients without CVD events, and FRS was better at predicting CVD events at 5 and 10 years. Therefore, PCE and D:A:D may be more useful in detecting HIV+ patients with low CVD risk.42
In addition to the screening tools discussed, biomarkers, such as certain interleukins, also appear to have a significant potential role in identifying HIV+ patients at increased risk for developing CVD.
Ultimately, the potential therapeutic role of biomarkers cannot be underestimated, and CVD risk is likely to be best estimated when biomarkers are assessed in combination. Thus, further research must go into studying current and identifying new biomarkers to improve the care of HIV+ patients.
Management and Switching ART Regimens
Treating traditional risk factors, such as hypertension and dyslipidemia, should be a mainstay for all patients. Avoiding ART with high CVD risk, and earlier diagnosis and treatment of HIV infection, will lower the burden of CVD among HIV+ patients over time.43
Multidisciplinary lifestyle interventions may be easily implemented in primary care visits for HIV+ patients and work to achieve smoking cessation along with improved diet and exercise, which may result in lower cholesterol levels. Yet, medications also have a role because lifestyle modification does not prevent all aspects of CVD risk, such as CIMT.44
The effectiveness of statins in treating dyslipidemia is well known; however, HIV+ patients diagnosed with their first acute coronary syndrome are less frequently prescribed a high-intensity statin in 3 years of follow-up compared with post-acute coronary syndrome HIV-negative patients. Even when statin therapy is prescribed for this population of HIV+ patients, they achieve less of a reduction in LDL cholesterol level and are less likely to reach cholesterol goals (except HDL cholesterol) at 6 months. Steps need to be taken to improve the lipid profiles of HIV+ patients to make them less atherogenic.45
For HIV+ patients who already have had a CVD event, consideration should be given to invasive procedures. Patients with HIV receiving coronary intervention experience no difference in mortality or cardiac death 1 to 3 years after the procedure.47 When examining percutaneous coronary intervention trends from 2003 to 2013 in HIV+ patients presenting with ST-elevation myocardial infarction, an increase in the proportions of this procedure and of drug-eluting stents is noted. Despite these facts, HIV+ patients are less likely to receive drug-eluting stents and more likely to receive bare metal stents than their HIV-negative counterparts.48
In HIV+ patients who have achieved virologic suppression, careful consideration must be given to the potential negative impact on metabolic and cardiovascular health prior to switching ART regimens. Several recent studies have looked at the effects of switching virologically suppressed HIV+ patients from an NRTI regimen of tenofovir disoproxil fumarate to tenofovir alafenamide.
In addition to NRTIs, recent studies also have evaluated the effects of integrase strand transfer inhibitor regimens on cardiovascular health.
Conclusion
People living with HIV have a higher risk for specific age-related health problems, especially CVD. The gap in CVD care between the general population and HIV+ patients must be closed, and as the knowledge base continues to grow, new ways to address this issue will arise. As the relationship between HIV infection, ART, traditional risk factors, and CVD becomes better understood, further research needs to be completed to determine the optimal CVD screening methods, management, and appropriate therapeutic interventions for the HIV+ population.
References
- Siddiqi AE, Hall HI, Hu X, et al. Population-based estimates of life expectancy after HIV diagnosis: United States 2008-2011. J Acquir Immune Defic Syndr. 2016;72(2):230-236.
- Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated individuals in the United States and Canada. PLoS One. 2013;8(12):e81355.
- Smit M, Cassidy R, Cozzi-Lepri A, et al. Projections of non-communicable disease and health care costs among HIV-positive persons in Italy and the U.S.A.: a modelling study. PLoS One. 2017;12(10):e0186638.
- Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117(2):214-220.
- Rodger AJ, Lodwick R, Schechter M, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27(6):973-979.
- Womack JA, Chang CC, So-Armah KA, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3(5):e001035.
- Cowell A, Shenoi SV, Kyriakides TC, et al. Trends in hospital deaths among human immunodeficiency virus-infected patients during the antiretroviral therapy era, 1995 to 2011. J Hosp Med. 2015;10(9):608-614.
- Paisible AL, Chang CC, So-Armah KA, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68(2):209-216.
- Levy ME, Greenberg AE, Hart R, et al. High burden of metabolic comorbidities in a citywide cohort of HIV outpatients: evolving health care needs of people aging with HIV in Washington, DC. HIV Med. 2017;18(10):724-735.
- Mayer KH, Loo S, Crawford PM, et al. Excess clinical comorbidity among HIV-infected patients accessing primary care in US community health centers. Public Health Rep. 2017:33354917748670.
- Koethe JR, Jenkins CA, Lau B, et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32(1):50-58.
- Helleberg M, May MT, Ingle SM, et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS. 2015;29(2):221-229.
- Fitch KV, Looby SE, Rope A, et al. Effects of aging and smoking on carotid intima-media thickness in HIV-infection. AIDS. 2013;27(1):49-57.
- Webel AR, Perazzo J, Longenecker CT, et al. The influence of exercise on cardiovascular health in sedentary adults with human immunodeficiency virus. J Cardiovasc Nurs. 2018;33(3):239-247.
- Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis. 2015;61(4):640-650.
- Janjua SA, Staziaki PV, Szilveszter B, et al. Presence, characteristics, and prognostic associations of carotid plaque among people living with HIV. Circ Cardiovasc Imaging. 2017;10(10):e005777.
- O’Dwyer EJ, Bhamra-Ariza P, Rao S, et al. Lower coronary plaque burden in patients with HIV presenting with acute coronary syndrome. Open Heart. 2016;3(2):e000511.
- Crowe SM, Westhorpe CL, Mukhamedova N, et al. The macrophage: the intersection between HIV infection and atherosclerosis. J Leukoc Biol. 2010;87(4):589-598.
- Khera AV, Cuchel M, la Llera-Moya de M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127-135.
- Maisa A, Hearps AC, Angelovich TA, et al. Monocytes from HIV-infected individuals show impaired cholesterol efflux and increased foam cell formation after transendothelial migration. AIDS. 2015;29(12):1445-1457.
- Hsue PY, Waters DD. Heart failure in persons living with HIV infection. Curr Opin HIV AIDS. 2017;12(6):534-539.
- Ogunmodede JA, Kolo PM, Katibi IA, et al. Structural echocardiographic abnormalities seen in HIV/AIDS patients are independent of cd4 count. Niger J Clin Pract. 2017;20(6):716-723.
- Nduka C, Sarki A, Uthman O, et al. Impact of antiretroviral therapy on serum lipoprotein levels and dyslipidemias: a systematic review and meta-analysis. Int J Cardiol. 2015;199:307-318.
- Nsagha DS, Weledji EP, Assob NJ, et al. Highly active antiretroviral therapy and dyslipidemia in people living with HIV/AIDS in Fako Division, South West Region of Cameroon. BMC Cardiovasc Disord. 2015;15:95.
- Abebe M, Kinde S, Belay G, et al. Antiretroviral treatment associated hyperglycemia and dyslipidemia among HIV infected patients at Burayu Health Center, Addis Ababa, Ethiopia: a cross-sectional comparative study. BMC Res Notes. 2014;7:380.
- Limas TG, Pinto Gde A, Marcato LM, et al. Analysis of the prevalence of dyslipidemia in individuals with HIV and its association with antiretroviral therapy. Rev Soc Bras Med Trop. 2014;47(5):547-551.
- Taramasso L, Ricci E, Menzaghi B, et al. Weight gain: a possible side effect of all antiretrovirals. Open Forum Infect Dis. 2017;4(4):ofx239.
- Abrahams Z, Levitt N, Lesosky M, et al. Changes in body fat distribution on dual-energy X-ray absorptiometry in black South Africans starting first-line antiretroviral therapy. AIDS Patient Care STDs. 2016;30(10):455-462.
- Dorjee K, Baxi SM, Reingold AL, et al. Risk of cardiovascular events from current, recent, and cumulative exposure to abacavir among persons living with HIV who were receiving antiretroviral therapy in the United States: a cohort study. BMC Infect Dis. 2017;17(1):708.
- Piconi S, Pocaterra D, Rainone V, et al. Maraviroc reduces arterial stiffness in PI-treated HIV-infected patients. Sci Rep. 2016:6:28853.
- Gatell JM, Assoumou L, Moyle G, et al. Switching from a boosted protease inhibitor (PI/r) based regimen to a dolutegravir regimen in virologically suppressed patients with high cardiovascular risk or age ≥50 years is non-inferior and decreases lipids. Presented at: 9th IAS Conference on HIV Science; July 23-26, 2017; Paris, France. Abstract TUAB0102.
- Ladapo JA, Richards AK, DeWitt CM, et al. Disparities in the quality of cardiovascular care between HIV-infected versus HIV-uninfected adults in the United States: a cross-sectional study. J Am Heart Assoc. 2017;6(11):e007107.
- Todd JV, Cole SR, Wohl DA, et al. Underutilization of statins when indicated in HIV-seropositive and seronegative women. AIDS Patient Care STDs. 2017;31(11):447-454.
- Molas E, Luque S, Retamero A, et al. Frequency and severity of potential drug interactions in a cohort of HIV-infected patients identified through a multidisciplinary team. HIV Clin Trials. 2018;19(1):1-7.
- McNicholl IR, Gandhi M, Hare CB, et al. A pharmacist-led program to evaluate and reduce polypharmacy and potentially inappropriate prescribing in older HIV-positive patients. Pharmacotherapy. 2017;37(12):1498-1506.
- Vos AG, Hulzebosch A, Grobbee DE, et al. Association between immune markers and surrogate markers of cardiovascular disease in HIV positive patients: a systematic review. PLoS One. 2017;12(1):e0169986.
- Vos AG, Idris NS, Barth RE, et al. Pro-inflammatory markers in relation to cardiovascular disease in HIV infection: a systematic review. PLoS One. 2016;11(1): e0147484.
- Raggi P, Zona S, Scaglioni R, et al. Epicardial adipose tissue and coronary artery calcium predict incident myocardial infarction and death in HIV-infected patients. J Cardiovasc Comput Tomogr. 2015;9(6):553-558.
- Phan BAP, Weigel B, Ma Y, et al. Utility of 2013 American College of Cardiology/American Heart Association cholesterol guidelines in HIV-infected adults with carotid atherosclerosis. Circ Cardiovasc Imaging. 2017;10(7):e005995.
- Krikke M, Hoogeveen RC, Hoepelman AI, et al. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic COronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) risk prediction models. HIV Med. 2016;17(4):289-297.
- Thompson-Paul AM, Lichtenstein KA, Armon C, et al. Cardiovascular disease risk prediction in the HIV outpatient study. Clin Infect Dis. 2016;63(11):1508-1516.
- Raggi P, De Francesco D, Manicardi M, et al. Prediction of hard cardiovascular events in HIV patients. J Antimicrob Chemother. 2016;71(12):3515-3518.
- Smit M, van Zoest RA, Nichols BE, et al. Cardiovascular disease prevention policy in HIV: recommendations from a modelling study. Clin Infect Dis. 2018;66(5):743-750.
- Saumoy M, Alonso-Villaverde C, Navarro A, et al. Randomized trial of a multidisciplinary lifestyle intervention in HIV-infected patients with moderate-high cardiovascular risk. Atherosclerosis. 2016;246:301-308.
- Boccara F, Miantezila Basilua J, Mary-Krause M, et al. Statin therapy and low-density lipoprotein cholesterol reduction in HIV-infected individuals after acute coronary syndrome: results from the PACS-HIV lipids substudy. Am Heart J. 2017;183:91-101.
- Ciaffi L, Cavassini M, Genne D, et al. Switch to etravirine for HIV-positive patients receiving statin treatment: a prospective study. Eur J Clin Invest. 2015;45(7):720-730.
- Bundhun PK, Pursun M, Huang WQ. Does infection with human immunodeficiency virus have any impact on the cardiovascular outcomes following percutaneous coronary intervention?: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2017;17(1):190.
- Albaeni A, Harris C, Eid SM, et al. HIV status and type of coronary stent placed in patients presenting with ST-elevation myocardial infarction. Coron Artery Dis. 2017;28(3):239-245.
The authors reported no relevant financial relationships.
Copyright © 2019 McMahon Publishing, 545 West 45th Street, New York, NY 10036. Printed in the USA. All rights reserved, including the right of reproduction, in whole or in part, in any form.
Download to read this article in PDF document:![]() Matters of the HEART: Understanding the Effect of HIV Treatment on Cardiovascular Health
Matters of the HEART: Understanding the Effect of HIV Treatment on Cardiovascular Health